-
ClearPoint Neuro, Inc. Announces License and Research Agreement with Philips
ソース: Nasdaq GlobeNewswire / 18 2 2021 15:05:00 America/Chicago
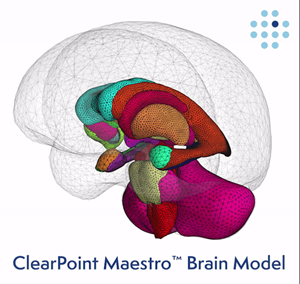
Collaboration will Enable ClearPoint to Develop and Launch the ClearPoint ‘Maestro™’ Brain Model by 2022
IRVINE, Calif., Feb. 18, 2021 (GLOBE NEWSWIRE) -- ClearPoint Neuro, Inc. (Nasdaq: CLPT) (the “Company”), a global therapy-enabling platform company providing navigation and delivery to the brain, today announced a worldwide license and research agreement with Philips, a global leader in health technology, to commercialize the ClearPoint ‘Maestro’ Brain Model. The company expects the first-generation anatomical segment analysis tool to be launched in 2022.
“This collaboration with Philips creates the foundation for all of our advanced software tools moving forward,” commented Joe Burnett, President and CEO of ClearPoint Neuro. “There are applications for Maestro across all of our current use cases. In drug delivery, we expect to automatically plan the safest and most efficient path to the target, and to quantify the infusion peri-procedurally to ensure patients receive the proper dose and coverage before they are closed and sent home. For deep brain stimulation, we aim to confirm lead placement and direction within the sub-nuclei. For laser ablation, we plan to identify eloquent structures of the brain to ensure heating does not damage crucial anatomy. For biopsies, we expect to identify and display tumor boundaries to take samples from the target region. Maestro will be the engine powering our new applications for years to come.”
“Philips aims to improve the health and well-being of people through meaningful innovation,” said Erik Pastink, Program Leader HealthTech IP Licensing at Philips IP&S. “By adopting an open innovation approach, we leverage our technology and intellectual property to bring innovations to the market in an effective manner, either through a Philips proposition, or that of a partner. The collaboration with ClearPoint Neuro where we combine our Brain Model technology with their Maestro solution is a great proof point of the latter approach.”
The Philips Brain Model which will be used in ‘Maestro’ emerged over 10 years ago from research aimed at detecting subtle volumetric and shape abnormalities in patients with mild traumatic brain injury. That first study was featured on the cover of the Journal of Neurotrauma. The unique methodology of the brain model combines deformable surfaces with active shape models and machine learning. More importantly, it provides point-based correspondence longitudinally and across patients. Cross-validation on more than 1,000 scans demonstrate highly reproducible results with sub-millimeter accuracy and normative values from 560 healthy subjects provide reference ranges for patient-specific assessments.
About ClearPoint Neuro
ClearPoint Neuro’s mission is to improve and restore quality of life to patients and their families by enabling therapies for the most complex neurological disorders with pinpoint accuracy. Applications of the Company’s current product portfolio include deep-brain stimulation, laser ablation, biopsy, neuro-aspiration, and delivery of drugs, biologics, and gene therapy to the brain. The ClearPoint Neuro Navigation System has FDA clearance, is CE-marked, and is installed in over 60 active clinical sites in the United States, Canada, and Europe. The Company’s SmartFlow® cannula is being used in partnership or evaluation with 25 individual biologics and drug delivery companies in various stages from preclinical research to late-stage regulatory trials. To date, more than 4,000 cases have been performed and supported by the Company’s field-based clinical specialist team which offers support and services for our partners. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
Statements herein concerning the Company’s plans, growth and strategies may include forward-looking statements within the context of the federal securities laws. Statements regarding the Company's future events, developments and future performance, as well as management's expectations, beliefs, plans, estimates or projections relating to the future, are forward-looking statements within the meaning of these laws. Uncertainties and risks may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: the impact of COVID-19 and the measures adopted to contain its spread; future revenues from sales of the Company’s ClearPoint Neuro Navigation System products; the Company’s ability to market, commercialize and achieve broader market acceptance for the Company’s ClearPoint Neuro Navigation System products; and estimates regarding the sufficiency of the Company’s cash resources. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2019, and the Company’s Quarterly Report on Form 10-Q for the three months ended September 30, 2020, both of which have been filed with the Securities and Exchange Commission, and the Company’s Annual Report on Form 10-K for the year ended December 31, 2020, which the Company intends to file with the Securities and Exchange Commission on or before March 31, 2021.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/088f3809-a329-47e4-bfc0-21655bb9e1b2
https://www.globenewswire.com/NewsRoom/AttachmentNg/03547784-c3a1-47ff-a8e6-63c5b5d9f152
Contact: Jacqueline Keller, Vice President, Marketing (949) 900-6833 info@clearpointneuro.com
ClearPoint Maestro Brain Model
ClearPoint Neuro, Inc. Announces License and Research Agreement with Philips. Collaboration will Enable ClearPoint to Develop and Launch the ClearPoint ‘Maestro™’ Brain Model by 2022
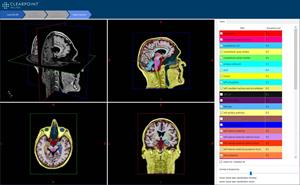
ClearPoint Maestro Brain Model Screenshot
Screenshot of the ClearPoint Maestro Brain Model, currently under development.